Functionalized surfaces
Functionalization is a special case of surface modification carried out for the purpose of giving a specific property (a function) to the surface. The formation of a layer of thiol containing molecules on gold nanoparticles is an important example of surface modification [1]. Surface functionalization can be realized by physical or chemical means, examples of both methods are illustrated here and in the linked pages. For instance, a steel sheet becomes rustproof after galvanization, namely deposition of a thin zinc layer on its surface.
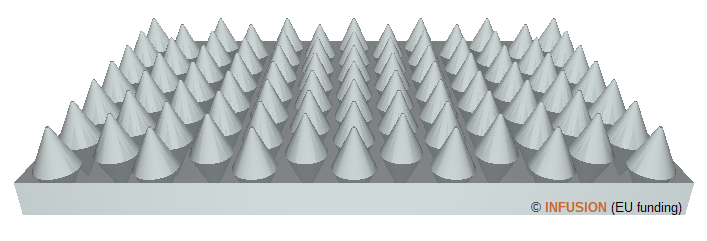 |
The left-hand side image is a computer rendering of a surface covered by a 2D periodic lattice of cones. When decorated with nanometer sized cones, the surface can acquire a superhydrophobic function. It means that the wettability of the surface by water is very small. A well-known natural example is the lotus leaf on which water droplets does not stick at all due to a particular sub-micrometer structure of the leaf surface. The structure illustrated on the left-hand side is predicted to be superhydrophobic in some range of the geometrical parameters such that the nanoscopic corrugation strongly reduces the Van der Waals interaction between the surface and a water droplet [2]. |
|
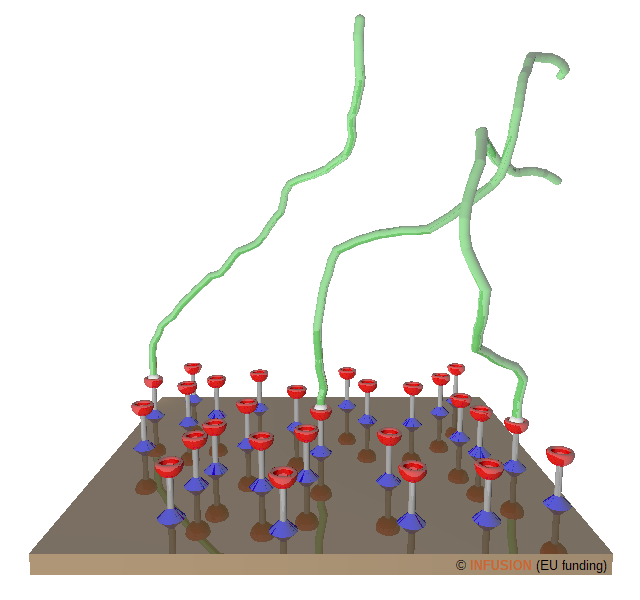
Grafting a polymer network onto a solid is a widely used technique to tailor surface properties, such tribology, corrosion, wetting ... [3]. The first step in the making of a so-called polymer brush consists in anchoring initiators (for instance alkyl bromides) on a functionalizable surface (silicon, gold, titanium ...). These molecules are schematized by the blue, gray and red objects standing on the surface. Eventually, an initiator becomes sort of a tether that binds a polymer chain to the surface by one of the chain ends (white ball in the figure). For clarity of the drawing, only three polymer chains have been represented (green features). |
 |
- "The Chemistry of the sulfur–gold interface: in search of a unified model" E. Pensa, E. Cortés, G. Corthey, P. Carro, C. Vericat, M.H. Fonticelli, G. Benítez, A.A. Rubert, and R.C. Salvarezza, Acc. Chem. Res. 45 (2012) 1183-1192 [DOI: 10.1021/ar200260p].
- "Quantum vacuum photon modes and superhydrophobicity" L. Dellieu, O. Deparis, J. Muller, and M. Sarrazin, Phys. Rev. Lett. 114 (2015) 024501 [DOI: 10.1103/PhysRevLett.114.024501].
- "50th anniversary perspective: polymer brushes: novel surfaces for future materials" W.L. Chen, R. Cordero, H. Tran, and C.K. Ober, Macromolecules 50 (2017) 4089-4113 [DOI: 10.1021/acs.macromol.7b00450].