Functionalized graphene
Perfect graphene is weakly reactive and has a small density of charge carriers available. Graphene chemistry would therefore be limited without the presence of defects, including the graphene borders, and chemical impurities. Both improve the reactivity locally. For some applications, it is preferable and often more easy to modify the surface of graphene rather than its structure.
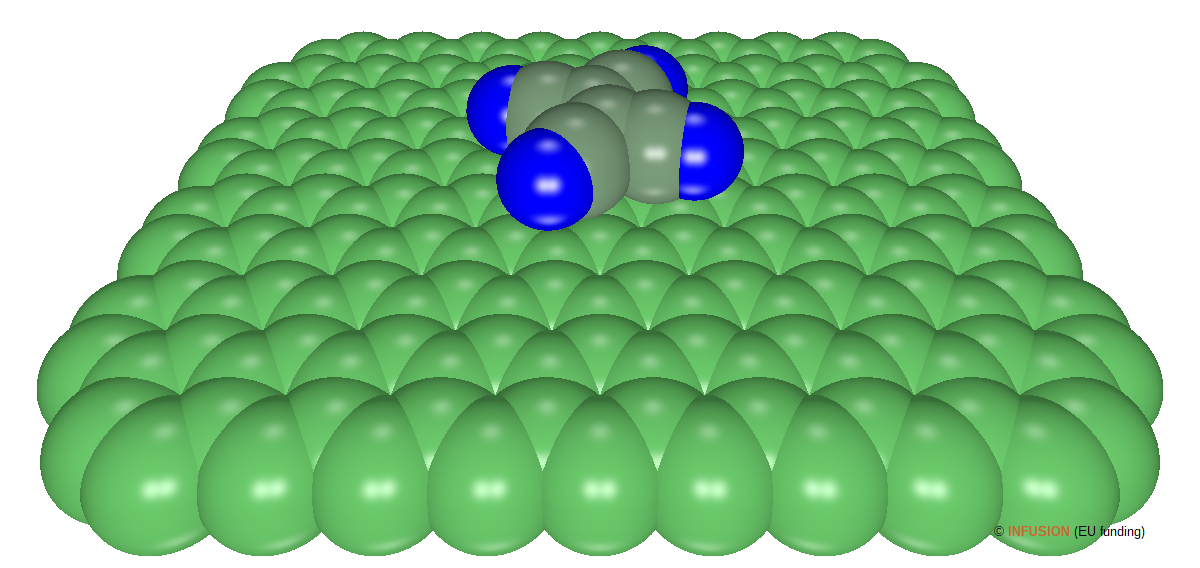 |
Doping graphene with electrons of holes can be obtained via charge transfer from or to adsorbed molecules. In case of non-covalent bonding, the structural and physical properties of graphene are weakly affected, which can be a great advantage [1]. Tetracyanoethylene (TCNE) C2(CN)4 is an example of an organic molecule that attracts electrons from graphene, while floating at van der Waals distance from the atomic plane of graphene. The image of the left-hand side is a 3D rendering of a TCNE molecule lying parallel to graphene and centered 0.31 nm above the center of an hexagon [2]. The atoms have been represented by van der Waals spheres (C in green, N in blue). |
|
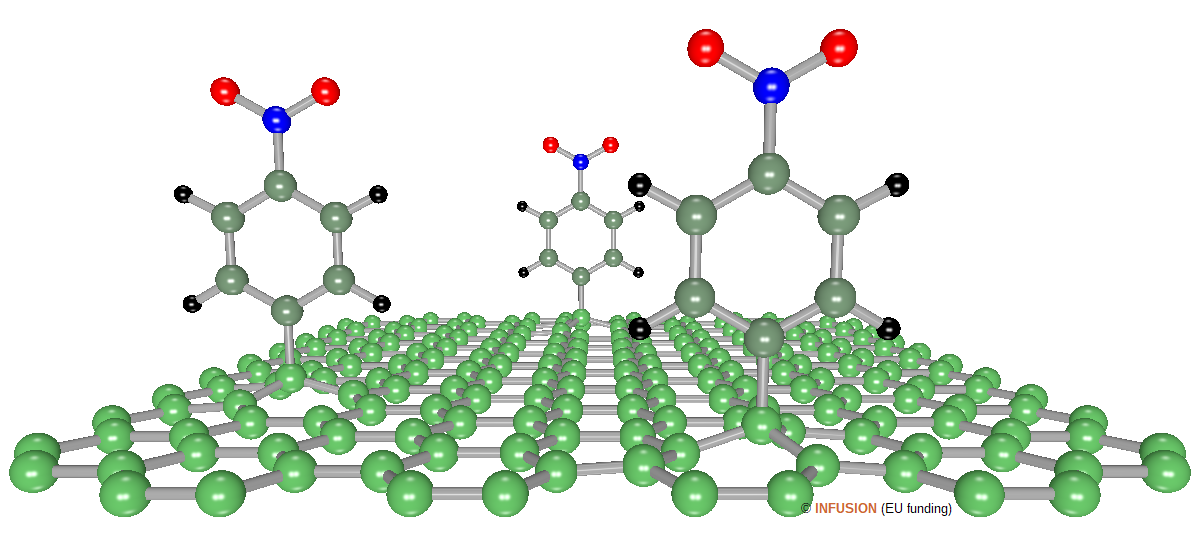
Covalent modification is essential for chemistry of graphene. In particular, a chemical treatment of graphene with aryl diazonium salts may lead to the attachment of organic molecules such as aromatic amines on the sp2 C network. The most plausible pathway of the reaction involves an electron transfer from graphene to the aryl diazonium cation, the release of a nitrogen molecule and covalent bonding of the aryl radical to a C atom [3]. This treatment opens the way to a variety of applications that would be impossible to achieve with pure graphene. Nowadays, diazonium chemistry has became an important route to tailor the electronic properties of graphene and reduce graphene oxide [4]. The figure illustrates the case of nitrobenzene (H5C6-NO2) functionalized graphene. After such a modification of its surface, few-layer graphene becomes semiconductor [5]. The C atoms on which the benzene rings are bound to are pulled out of the plane of the graphene network due to a change of hybridization from sp2 to sp3. |
 |
- "Non-covalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications" V. Georgakilas, J.N. Tiwari, K.C. Kemp, J.A. Perman, A.B. Bourlinos, K.S. Kim, and R. Zboril, Chem. Rev. 116 (2016) 5464-5519 [DOI: 10.1021/acs.chemrev.5b00620].
- "TCNE-modified graphene as an adsorbent for N2O molecule: a DFT study" S.F. Rastegar and N. Osouleddini, J. Mol. Model. 23 (2017) 352 [DOI: 10.1007/s00894-017-3526-2].
- "Covalent electron transfer chemistry of graphene with diazonium salts" G.L.C. Paulus, Q.H. Wang, and M.S. Strano, Acc. Chem. Res. 46 (2013) 160–170 [DOI: 10.1021/ar300119z].
- "Impact of covalent functionalization by diazonium chemistry on the electronic properties of graphene on SiC" G. Ambrosio, A. Brown, L. Daukiya, G. Drera, G. Di Santo, L. Petaccia, S. De Feyter, L. Sangaletti, and S. Pagliara, Nanoscale 12 (2020) 9032-9037 [DOI: 10.1039/D0NR01186J].
- "Chemical modification of epitaxial graphene: spontaneous grafting of aryl groups" E. Bekyarova, M.E. Itkis, P. Ramesh, C. Berger, M. Sprinkle, W.A. de Heer, and R.C. Haddon, J. Am. Chem. Soc. 131 (2009) 1336-1337 [DOI: 10.1021/ja8057327].