Definition
In physics, chemistry and biology, self-assembly refers to a process by which the entities of a system spontaneously arrange in an ordered pattern. From the thermodynamic point of view, this means that the self-assembled structure is more stable than any non-ordered arrangement of the entities. The entities are molecules or atomic clusters, or still more complex structures. Unlike in most crystals, the ordering is governed by non-covalent, non-ionic and non-metallic interactions, such as van der Waals force, π-π stacking, hydrogen bonds, electrostatics interactions, and others [1].
A well-known example of self-assembled structure is opal. Precious opal is a close-packed assembly of small hydrated silica spherulites presenting a narrow dispersion of diameter, which averages typically around 0.25 μm. A remarkable characteristics of opal is its space filling fraction, which can approach the largest possible value for mono-disperse spheres, 0.74. After a long chemico-physical process, the stacked micro-spherulites are cemented together to form a solid. Opal is a natural photonic crystal for visible radiations due to the close matching of the SiO2 spherulite diameter and wavelength. Most of the color effects precious opal is generating come from diffraction and interference phenomena. Artificial opal can be fabricated from colloids of various materials, including polymers, such as polystyrene and poly methyl methacrylate.
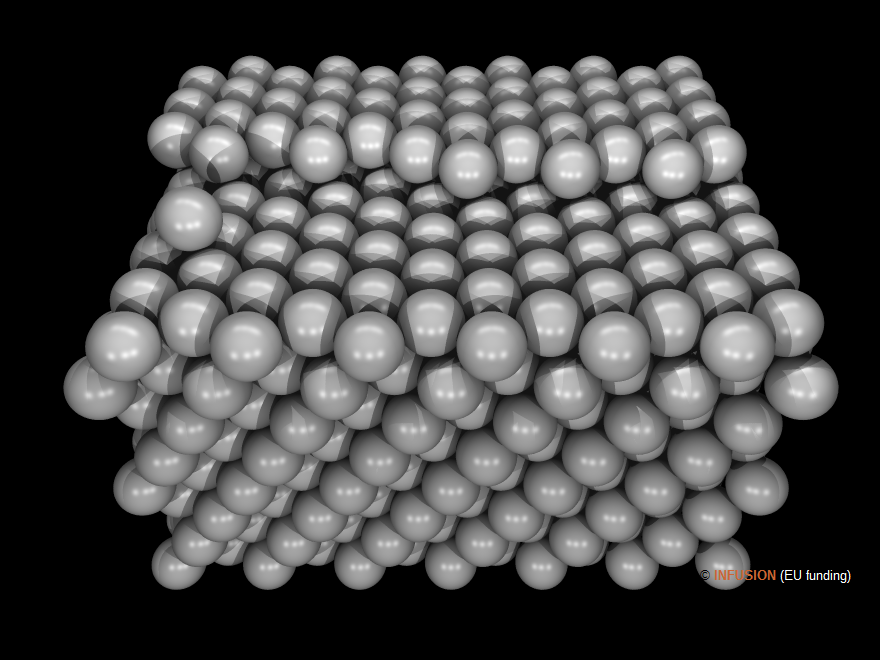 |
Computer model of precious opal with its closed-packed layers piled up in the vertical direction. |
- "Beyond molecules: self-assembly of mesoscopic and macroscopic components" G.M. Whitesides and M. Boncheva, Proc. Nat. Acad. Sci. USA. 99 (2002) 4769-4774 [DOI: 10.1073/pnas.082065899].