Modified carbon nanotubes
The functionalization of carbon nanostructures can modify their chemical-physical properties to gain control on their interactions, ultimately mastering their organization. This control may be of particular importance in the making of polymer composites loaded with carbon nanotubes. Suitable functional groups attached to the sidewall of the nanotubes may reduce their tendency to agglomerate, hence improving the homogeneity of the composite, and increase their adherence to the polymer matrix. Many other applications of nanotubes, starting from solubility, rests on chemical functionalization [1].
|
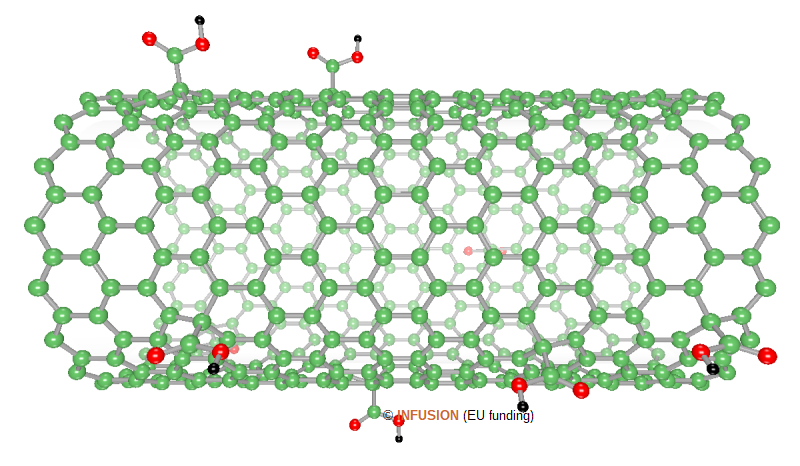
The image on the right-hand side represents a single-wall (18,0) carbon nanotube decorated with a few carboxyl O=C–OH groups (O atoms as red balls, H atoms as black dots). In the present representation, the percentage of carboxylic functions is 1.6 %. These functional groups can be covalently bound to an ending cap of a nanotube. The attachment of a carboxyl group on a sidewall atom involves a change of sp2 to sp3 hybridization, which requires the presence of a defect. For that reason, a multiwall nanotube lends itself well to this type of modification. Once fixed on a nanotube, a carboxyl group can serve to tether different types of chemical moieties [2]. For instance, decoration with sticky moieties, like hydrogen bonding donor/acceptor groups, and their interaction with complementary photoactive moieties may allow the formation of composite materials with a broad spectrum of photophysical properties. |
 |
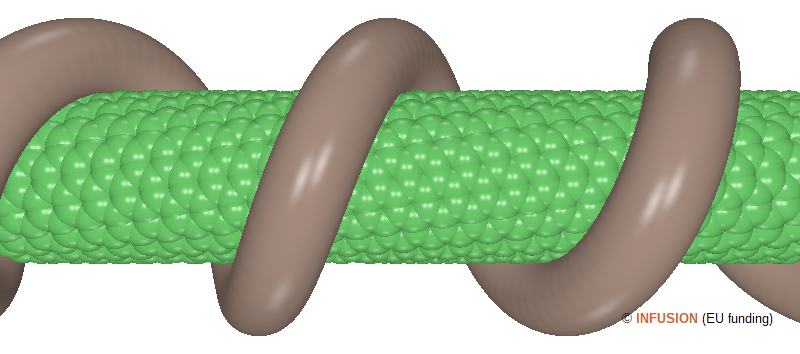 |
Unlike the previous example, the image on the left-hand side illustrates a case of functionalized nanotube by non-covalent bonding. Organic molecules and surfactants can stick on the surface of a carbon nanotube by weak links, such as π stacking and hydrophobic interactions. The image schematizes the wrapping of a polymer chain around a (12,7) nanotube. Polyfluorene is an example of polymer that can do that. Among other benefits, the dispersion of nanotubes modified this way may be greatly improved in some organic solvents [3]. In the figure, The C atoms have been drawn with their van der Waals radius of 0.17 nm. The external surface of the nanotube looks like a corrugated cylinder of 0.82 nm radius (refer to the nanotube calculator). The wrapped polymer has been represented by a continuous helical structure (1.13 nm outer radius, 0.50 nm minor radius, and 2.73 nm pitch length). |
- "Chemically functionalized carbon nanotubes" K. Balasubramanian and M. Burghard, Small 1 (2005) 180-192 [DOI: 10.1002/smll.200400118].
- "Improved mechanical properties of carbon nanotube/polymer composites through the use of carboxyl-epoxide functional group linkages" S.H. Park and P.R. Bandaru, Polymer 51 (2010) 5071-5077 [DOI: 10.1016/j.polymer.2010.08.063].
- "Conjugated polymer-wrapped carbon nanotubes: physical properties and device applications" W. Gomulya, J. Gao, and M. Loi, Eur. Phys. J. B 86 (2013) 404-416 [DOI: 10.1140/epjb/e2013-40707-9].