Reduced graphene oxide
Graphene oxide (GO) and its reduced forms (reduced graphene oxide, rGO) can be considered as functionalized graphene. They are interesting materials by themselves [1]. Both are semiconducting materials whose band-gap depends on their actual structure [2] and both exhibit photoluminescence (PL), among other properties. The PL spectrum of GO depends on its degree of oxidation. The maximum of intensity shifts from 710 nm (red) in as-prepared GO to 450 nm (blue) in rGO [3].
|
The synthesis of GO and rGO follows a top-down cost-effective approach. GO is obtained by oxidation of small particles of graphite, which are next exfoliated. Compared to graphene, the GO structure has a lot of structural defects. It contains epoxide (C-O-C), hydroxyl (C-OH), carbonyl (C=O) and carboxyl (C(=O)-OH) groups attached on the surface and along the edges, including the edges of holes in the pseudo-graphitic network. rGO is often obtained from oxidized graphite rather than from GO by chemical exfoliation in solution followed by a treatment with a reducing agent, such as N2H4, or by a (photo)thermal process. Structural defects are partly repaired (around 70 at% C have sp2 hybridization) and the concentration of oxygen is reduced. The figure on the right-hand side is a schematic representation of rGO (oxygen atoms are represented in red, hydrogen in black). |
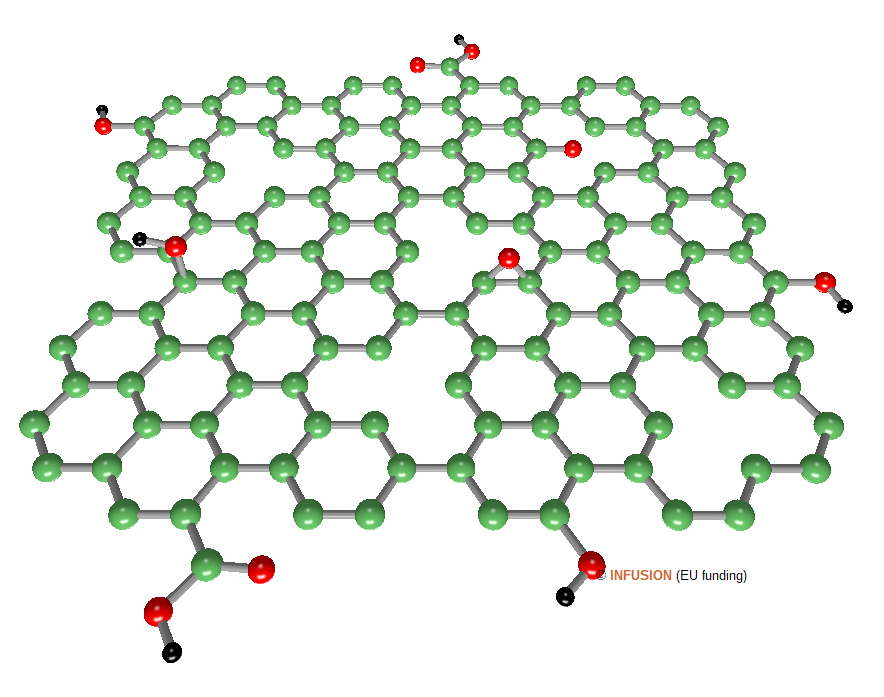 |
- "Reduced graphene oxide today" R. Tarcan, O. Todor-Boer, I. Petrovai, C. Leordean, S. Astilean, and I. Botiz, J. Mater. Chem. C 8 (2020) 1198-1224 [DOI: 10.1039/C9TC04916A].
- "Band gap of reduced graphene oxide tuned by controlling functional groups" Y. Jin, Y. Zheng, S.G. Podkolzin, and W. Lee, J. Mater. Chem. C 8 (2020) 4885-4894 [DOI: 10.1039/C9TC07063J].
- "Tunable photoluminescence from graphene oxide" C. Chien, S. Li, W. Lai, Y. Yeh, H. Chen, I. Chen, L. Chen, K. Chen, T. Nemoto, S. Isoda, M. Chen, T. Fujita, G. Eda, H. Yamaguchi, M. Chhowalla, and C. Chen, Angew. Chem. Int. Ed. 51 (2012) 6662-6666 [DOI: 10.1002/anie.201200474].