Introduction
The colors of an object seen by our eyes is produced by electromagnetic radiations from the visible range that are emitted, absorbed or reflected by it. By convention (CIE normalization), the visible spectrum encompasses radiations whose wavelength ranges from 380 nm to 780 nm. The color depends firstly on the characteristics of our eyes. Selective absorption of the incoming light by the three types of cone receptors on the retina generates stimuli that are interpreted as a color by the brain. Lower down the eyes, the color is controlled by the spectral distribution of the intensity of light.
The color of a black body (an ideal medium that absorbs all the incident radiations) depends on its temperature. If the wavelength of maximum intensity is inversely proportional to the temperature T (Wien's displacement law), the exact color of the black body depends on the intensity distribution (Planck's law) over the full visible spectrum. The following table compares the apparent colors for black-body temperatures between 3000 and 6000 K [John Walker, http://www.fourmilab.ch/]. The photosphere of the Sun is seen approximately like a black body at T=5800 K.

Strictly speaking, a spectrum refers to the frequency of the radiations, which is a more intrinsic characteristics than the wavelength. The latter indeed depends on the index of refraction of the medium in which light travels. In vacuum, the wavelength λ is related to the frequency ν by λ = c/ν with c = 299 792 458 m/s the velocity of light in vacuum.
The emission or absorption of visible radiations is the result of transitions between electronic states of atoms or molecules, or between electronic bands in polymers or solids, or comes from collective oscillations (plasmons) of free charges in metallic particles. The energy of the transition is directly related to the frequency of the radiations or their wavelength in vacuum by the Einstein relation E = hν = hc/λ. Here h is the Planck's constant.
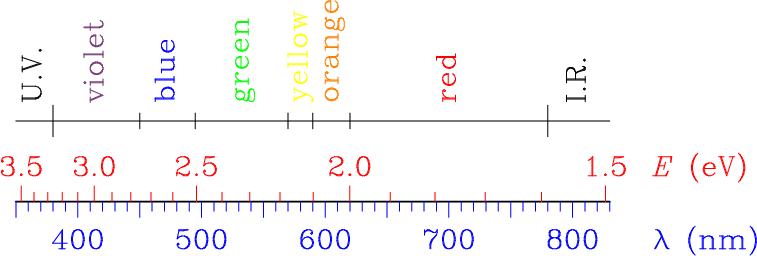 |
The diagram reflects the correspondence between wavelength in vacuum and energy for electromagnetic radiations in the interval (350,830) nm. The range of conventional colors is shown on the top part of the figure. I.R. and U.V. mean infra-red and ultraviolet, respectively. |
Two colors whose addition in equal intensity produces white are said complementary. The following table illustrates couples of complementary colors:
