Self-assembled monolayers (SAM)
Under appropriate circumstances, organic molecules adsorbed on a solid surface can be arranged in domains of well-ordered structures [1]. The obtained ordered monolayer is abbreviated as SAM. SAM can also be produced on the surface of nanoparticles.
In the simplest cases, the molecules have an anchoring group that bonds to the surface. When the density of molecules adsorbed on the surface is sufficient, the tails of neighboring adsorbed molecules interact with each other to adopt the same conformation. Often, the tail of the adsorbed molecules ends with a functional group on which other molecules can attach. For some application, fictionalization of the tail is used to change the surface properties, such as wetting, reactivity... . SAM are widely used in silicon-chip technology, molecular electronics, nanotechnology, for sensing or biological purposes etc.
A textbook example of SAM is provided by alkane-thiol chains adsorbed on gold, whose ordering can be visualized by STM imaging. After deprotonation resulting from the surface interactions, the S atom of each molecule bond to three atoms on a hollow site of the (111) surface. A √3×√3R superstructure is observed with hexagonal lattice parameter a = 0.50 nm. The tails of the molecular chains are oriented at 27° from the normal. Thanks to the tilt angle θ, the interchain distance is reduced to d = a cosθ = 0.446 nm, close to the normal van der Waals distance between alkane chains.
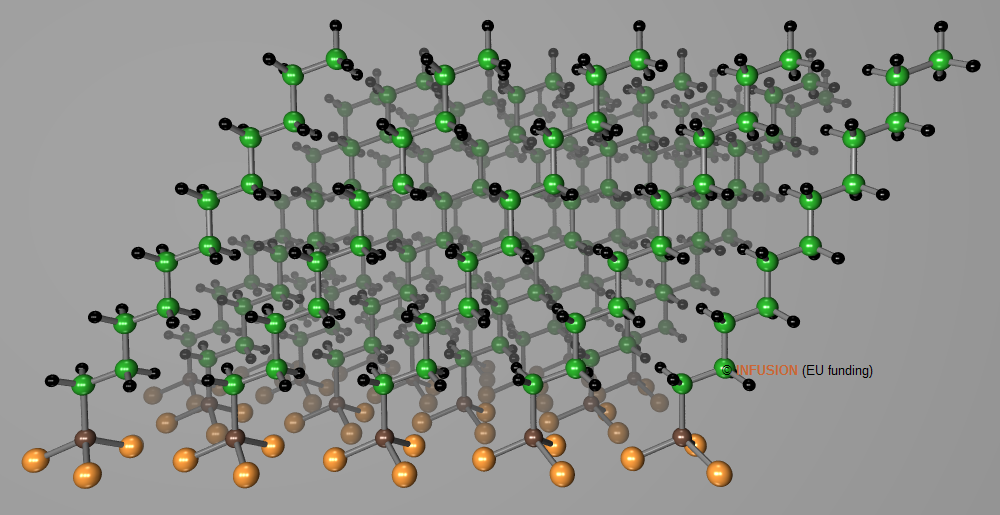 |
Computer representation of a dodecanethiol SAM on Au(111) formed by the adsorption of dodecanethiolate (C12H25S-) on the surface. The sulfur atoms are represented by brown spheres attached to three gold atoms. |
Among other examples, fatty acids self-assemble of Ag0 or Al2O3. Organosilicon derivatives can also form SAM on a variety of surfaces, such as quartz, glass, mica, oxidyzed silicon... .
- "Formation and structure of self-assembled monolayers" A. Ulman, Chem. Rev. 96 (1996) 1533-54 [DOI: 10.1021/cr9502357].