Introduction
Graphite is the most stable crystalline form of pure carbon in normal temperature and pressure conditions. Its crystallographic structure was determined by Bernal in 1924 by X-ray diffraction from a natural single crystal. It consists in a stacking along the c axis of what is now called graphene planes. In the Bernal structure, every other two graphene planes are shifted parallel to its neighboring layers by one CC distance (0.142 nm) along the diagonal of the 2D unit cell. This is the ABAB stacking of hexagonal graphite. ABCABC sequence can be found in the rhombohedral form, a minor component of graphite. With an inter-plane distance of 0.3354 nm, both forms have a density of 2.26 103 kg/m3.
|
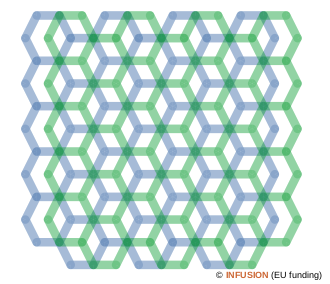
Schematic representation of the Bernal stacking in graphite. The graphene planes alternate along the c axis between the blue and green positions. There are columns of atoms (shown by 6-branch stars) that have direct neighbors up and down at the inter-plane distance. The vertical distance between the remaining atoms is twice the inter-plane separation. |
 |
It is only in 2004 that a single layer of graphene could be isolated from graphite and characterized as such by physical methods [1]. The promises of the new physics that was born then triggered a lot of attention of the scientific community for 2D materials. The importance of the discovery of graphene by physicists was recognized by the Nobel prize in physics attributed to Novoselov and Geim in 2010. Since then, other forms of 2D carbon structures have been either predicted or synthesized, or both. For some applications that do not require perfect structures, graphene can be replaced by reduced graphene oxide. Unlike graphene, the latter can be produced by cost-effective top-down chemistry.
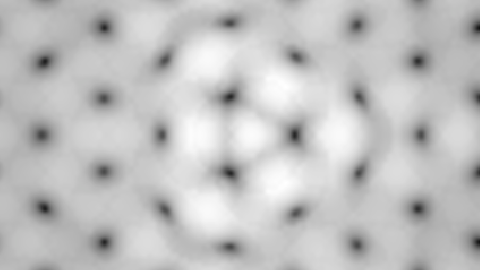 |
Computer-generated constant-current STM image of graphene containing one substitutional nitrogen impurity located at the center of the figure. The triangular shape of the pyridine-like defect is the result of a strong perturbation of the local electronic density of states induced by the impurity on its first and third neighbors C atom. |
- "Electric field effect in atomically thin carbon films" K.S. Novoselov, A.K. Geim, S.V. Morozov, D. Jiang, Y. Zhang, S.V. Dubonos, I.V. Grigorieva, and A.A. Firsov, Science 306 (2004) 666-669 [DOI: 10.1126/science.1102896].