Graphene
Graphene is the first real two-dimensional material ever isolated. Graphite, the common allotrop of pure carbon, is a stacking of a myriad of graphene planes bound by weak van der Waals forces. Graphene has a honeycomb structure, with lattice parameter 0.246 nm. Its covalent structure is formed by the σ overlap of the C sp2 hybrid orbitals. The electrons coming from the remaining 2pz orbitals are delocalized over the atomic plane. Their energy is distributed over two bands, respectively located below (bonding π band) and above (antibonding π* band) the Fermi level. Graphene is a zero-gap semiconductor that can easily be doped, intentionally or not, by external fields or by impurities and defects.
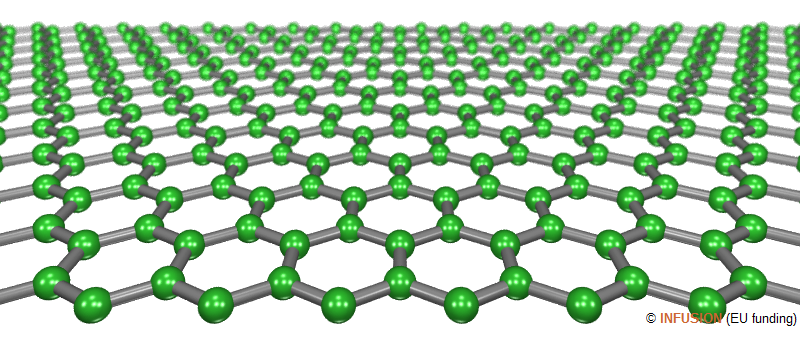 |
Ball-and-stick model of a graphene layer with a zigzag free edge in the foreground. Except at the edges, three bonds intersect at 120° on each atom. The bonds have a length of 0.142 nm. |
Graphene is the prototypical material of nanotechnology. It can serve as a basic unit of field-effect nanotransistors and in many other devices, such as sensors, transparent electrodes, photodetectors ... . It can be used as a ultrathin substrate on which molecular groups can attach or stick.
Physical properties of monolayer graphene
Lattice parameter: a = 0.246 nm
Reciprocal lattice parameter: b = 4π/√3a = 29.5 nm-1
Space group symmetry: P6/mmm
Mass density: 0.761 10-6 kg/m²
In-plane stiffness (Young) modulus [1]: 340 N/m
Bending stiffness [2]: 1.6 eV
Raman lines at 2.41 eV [2]: ωG = 1585 cm-1, ω2D = 2690 cm-1, ωG* = 2450 cm-1
- "Elastic properties and stability of physisorbed graphene" Ph. Lambin, Appl. Sci. 4 (2014) 282-304 [DOI: 10.3390/app4020282].
- "Raman spectroscopy in graphene" L.M. Malard, M.A. Pimenta, G.Dresselhaus, M.S. Dresselhaus, Phys. Rep. 473 (2009) 51-87 [DOI: 10.1016/j.physrep.2009.02.003].