Graphdiyne
Graphdiyne, produced and fully characterized in 2010 [1], is a periodic structure similar to graphyne, though more complex. It is composed of 6-membered carbon rings interconnected by linear linkage groups containing two triple bonds [2]. There are 18 atoms in the hexagonal unit cell with lattice parameter 0.946 nm. The planar density of atom is 23.32 nm-2 to be compared with 38.18 nm-2 for graphene. Graphdiyne can be synthesized by bottom-up organic chemistry routes [3]. However, numerous challenges remained to be solved when Ref [3] was published. A serious difficulty is to control the number of layers.
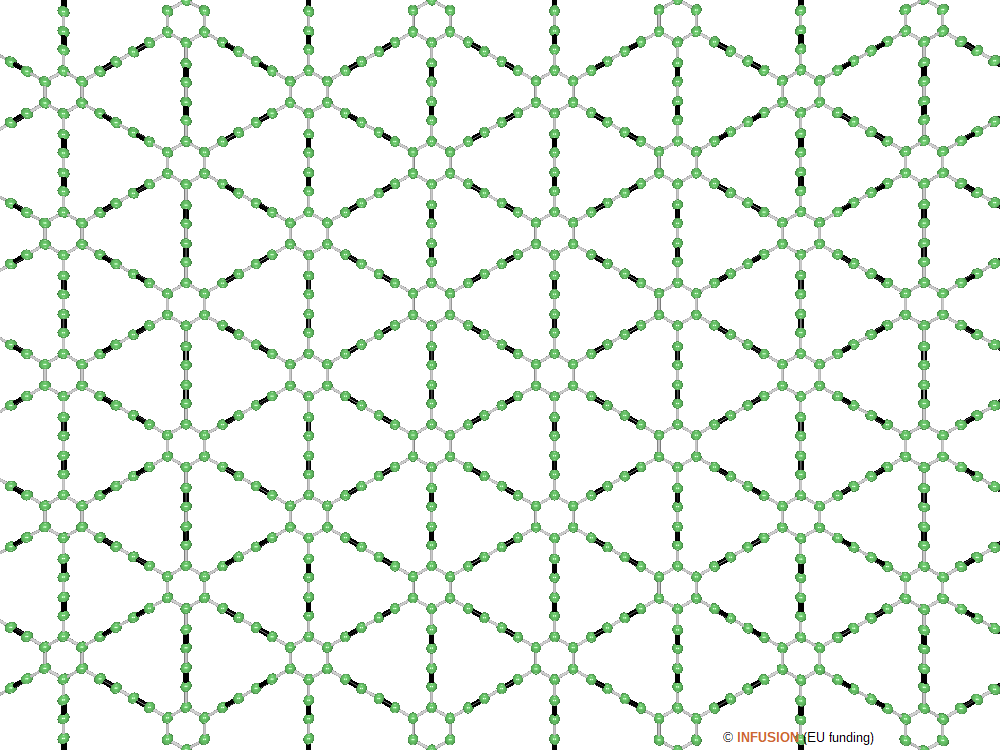 |
Structural model of graphdiyne with two triple bonds, represented in black, in each linkage chain. There are four type of bonds: the C-C bonds in the benzene ring with 0.143 nm length, the C-C bond that connects a linkage chain to a bezene, with length 0.140 nm, the triple bonds of length 0.123 nm, and the C-C bond of length 0.134 nm at the center of the chain. |
Graphdiyne combines almost all the potential properties of graphene plus other ones specific to its atomic or electronic structures. Unlike graphene, graphdiyne is permeable to small molecules, thanks to its triangular pores delimited by diacetylenic linkages. The van der Walls diameter of these pore is 0.63 nm, which makes graphdiyne membranes suitable for gas separation. Graphdiyne looks so promising in many fields that a special issue of the journal Advanced Materials has been devoted to it (volume 31, issue 42, October 2019).
According to state-of-the-art GW band-structure calculations, graphdiyne is a 1.1 eV direct bang gap semiconductor at the Γ point [4]. The optical bang gap is reduced to 0.75 eV by excitonic effects. By comparison, the photoluminescence spectrum presents a peak at 1.79 eV (693 nm). Nanoscopic quantum dots can be produced by an appropriate solvothermal treatment of a graphdiyne sheet. The photoluminescence spectrum of the obtained nanostructures is influenced by the existence of functional groups on their edges. It depends on the excitation wavelength and on the local pH, making it possible to tune the optical properties of the quantum dots [5].
- "Architecture of graphdiyne nanoscale films" G. X. Li, Y. L. Li, H. B. Liu, Y. B. Guo, Y. J. Li, and D. B. Zhu, Chem. Commun. 46, 3256 (2010) [DOI: 10.1039/b922733d].
- For a review, see: "Graphdiyne: synthesis, properties, and applications" X. Gao, H. Liu, D. Wang, and J. Zhang, Chem. Soc. Rev. 48 (2019) 908-936 [DOI: 10.1039/C8CS00773J].
- "Exploring approaches for the synthesis of few‐layered graphdiyne" J. Zhou, J. Li, Z. Liu, and J. Zhang, Adv. Mater. 31 (2019) 1803758 [DOI: 10.1002/adma.201803758].
- "Quasiparticle energies and excitonic effects of the two-dimensional carbon allotrope graphdiyne: Theory and experiment" L. Guangfu, Q. Xuemin, L. Huibiao, Q. Rui, Z. Jing, L. Linze, G. Zhengxiang, W. Enge, M. Wai-Ning, L. Jing, L. Yuliang, and S. Nagase, Phys. Rev. B 84 (2011) 075439 [DOI: 10.1103/PhysRevB.84.075439].
- "Synthesis and imaging of biocompatible graphdiyne quantum dots" H.M.,Yingqiu Qi, Y. Chen, Y. Zhang, X. Han, Y. Xu, Y. Liu, J. Hu, H. Liu, Y. Li, and G. Nie, Appl. Mater. Interf. 11 (2019) 32798-807 [DOI: 10.1021/acsami.9b12801]. .