Graphane
Graphane, also called bi-dimensional hydrocarbon, is a fully-hygrogenated graphene sheet [1]. Each hygrogen is bound to one carbon atom, with the hydrogens being located alternatively on one side and the other of the sheet. Different configurations have been predicted theoretically, the one shown in the figure below is called the chair structure [2].
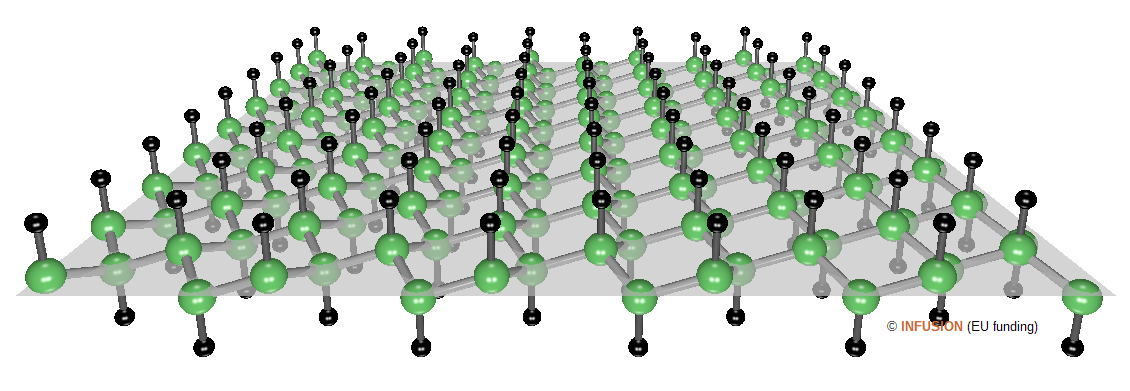 |
Ball-and-stick model of the chair configuration of graphane. The C-C distance is 0.154 nm and the C-H bond length is 0.111 nm. The C atoms are shited outside the symmetry plane by 0.023 nm. |
- "Control of graphene's properties by reversible hydrogenation: evidence for graphane" D.C. Elias, R.R. Nair, T.M.G. Mohiuddin, S. . Morozov, P. Blake, M.P. Halsall, A.C. Ferrari, D.W. Boukhvalov, M.I. Katsnelson, A.K. Geim, and K.S. Novoselov, Science 323 (2009) 610 [DOI: 10.1126/science.1167130].
- For a review, see "Graphene’s cousin: the present and future of graphane" C. Zhou, S. Chen, J. Lou, J. Wang, Q. Yang, C. Liu, D. Huang, and T. Zhu, Nanoscale Res. Lett. 9 (2014) 26 [DOI: 10.1186/1556-276X-9-26].