Transition metal oxides
Transition metal oxides and hydroxides, such as WO3, MoO3, V2O5, Nb2O5, Ir(OH), Co3O4, NiO, are among the first materials where electrochromism was observed. The prototypical example is tungsten trioxide that, in thin film, is transparent. When a small quantity of positive ions, such as Li+, coming from an electrolyte, are reversibly incorporated in the film together with electrons coming from an electrode, WO3 is transformed in a new compound, e.g. LixWO3. In this compound, a fraction x of the W atoms are reduced from the oxidation state WVI to the oxidation state WV (x may vary between 0 and 0.3, typically). The reduced film acquires a blue color due to light absorption that produces a transfer of electrons between adjacent WV and WVI sites. In contrast with WO3, Ir, Co and Ni oxides are colored in the oxidized state.
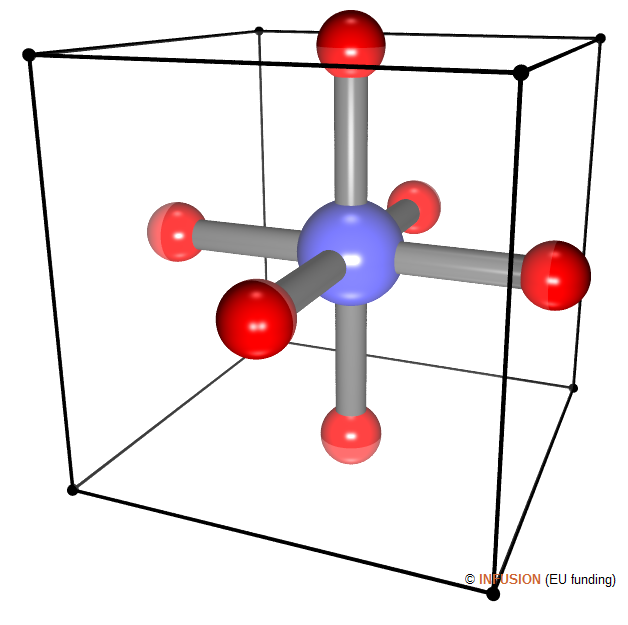 |
WO3 is a semiconductor. In the crystal, each W atom (blue sphere) bonds to six O atoms. These latter form an open framework of corner-sharing octahedra having W at their center [1]. Alkali atoms can be intercalated into the voids of the structure, at the location of the black dots in the opposite figure, where they donate electrons that start filling the conduction band. |
The intercalation of metal cations in an oxide film is a slow process. It means that switching between the transparent and colored states and back takes time. Transition metal oxides are therefore not suitable for display applications, but are good candidates for smart-window technology.
- "Structure and properties of WO3 thin films for electrochromic device application" M.C. Rao, J. of Non-Oxide Glasses 5 (2013) 1-8 [pdf].