Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) are molecules composed of carbon and hydrogen that comprise several aromatic rings. Their electronic properties are governed by π electrons delocalized over the six-numbered rings. PAHs can be obtained from coal and oil. They are present in the interstellar medium of our Galaxy and in other galaxies as well.
Charged PAHs can support plasmon resonances in the visible range. Accordingly, strong optical absorption can be induced in these molecules and reversibly switched on and off by adding and then removing an electron [1]. This property can be exploited in electrochromic devices, as demonstrated in ref [1]. The proof-of-concept device contained anthracene molecules dissolved in a conductive transparent gel sandwiched between two transparent electrodes. By applying a -4V voltage for a few seconds between the electrodes, the color of the gel changes from gray to blue and fades out when the voltage drops to 0.
|
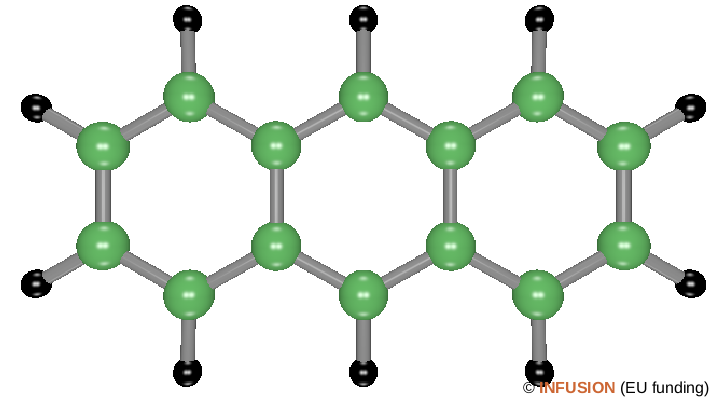
Ball-and-stick model of the linear anthracene molecule C14H10 that contains three fused benzene rings. In the neutral state, this molecule is transparent in the visible range (band gap of 3.7 eV located in the UV). In its -1 charged state, collective electron excitations take place upon visible light irradiation, which gives rise to a broad absorption band having its maximum at 1.71 eV (725 nm). |
 |
- "Molecular Plasmonics" A. Lauchner, A.E. Schlather, A. Manjavacas, Y. Cui, M.J. McClain, G.J. Stec, F.J. García de Abaj, P. Nordlander, and N.J. Halas, Nano Lett. 15 (2015) 6208–6214 [DOI: 10.1021/acs.nanolett.5b02549].