Polythiophenes
Polythiophenes are tiophene (C4H4S)-based polymers. Being conjugated systems, their pi electrons are largely delocalized along the skeleton of the chain. Undoped polythiophene is red and turns blue upon oxidation. The optical band gap of the undoped polymer can be modified by introducing different substituents onto the monomer units. In addition to the possibility they offer for color tuning, polythiophenes are interesting electrochromic organic materials due to their chemical stability.
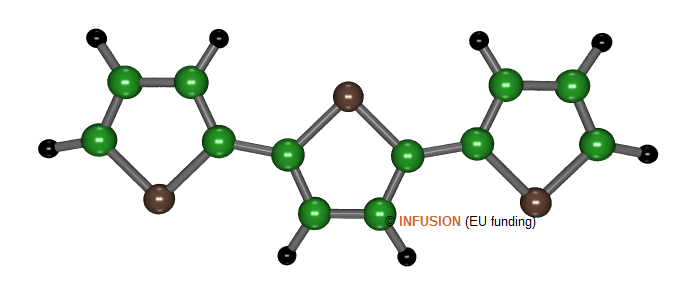 |
Atomic structure of tertiophene, an oligomer composed of three thiophene units. The sulfur atoms are represented in brown. |
Poly(3,4-ethylenedioxythiophene) (PEDOT) is an intrinsically conductive polymer used in a broad range of applications. The monomer is a thiophene with the two carbon atoms opposite to the sulfur being bridged by a dioxyethylene group (C2H2O2). The 1.6-eV band gap of neutral PEDOT is responsible for its blue color. In the oxidized state, the color of PEDOT turns to sky blue. It becomes black in the reduced state. Many other polymers based on modified EDOT monomers can be used as electrochrome materials.