Conducting polymers
Some polymers can be made conducting by chemical doping). Among several interesting properties, a conducting polymer can display electrochromism. In the undoped state, the polymer is not conducting. Depending on the magnitude of its HOMO-LUMO gap, it is either colored or transparent. In the doped state, electronic subbands appear in the HOMO-LUMO gap due to the acceptance (reduction) or donation (oxidation) of electrons by the polymer to the dopant species. This process create charge carriers somewhat delocalized over the polymer backbone [1]. As a result of new states in the HOMO-LUMO gap, the absorption band is redshifted, which can produces a visible change of color of the polymer. To trigger this property, dopant ions or protons need to be inserted and de-inserted reversibly by application of an electric voltage.
Polymers are solid but flexible materials that are used in the form of thin films in electrochromic devices. The solid state offers several obvious advantages compared to liquids or solutions for electrochromic applications.
Polyaniline is a conducting polymer whose unit cell derives from aniline C6H5NH2. Polyaniline can be viewed as a mixing of three compounds: leucoemeraldine (C6H4NH)n --transparent, emeraldine ([C6H4NH]2[C6H4N]2)n --green or blue, depending on the dopant-- and pernigraniline (C6H4N)n --blue or violet, depending on the dopant.
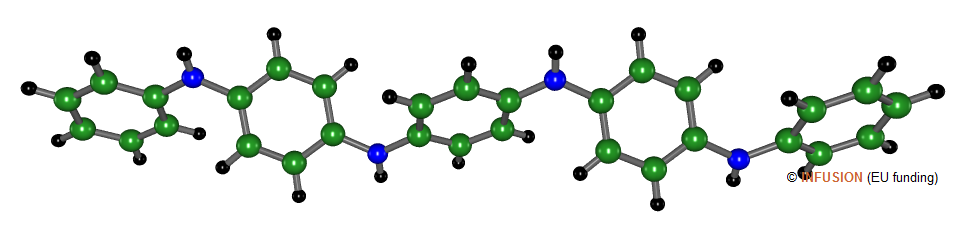
The molecule illustrated here is a pentamer of anniline (more precisely, a tetramer terminated at one end by a phenyl group) whose structure has been derived from a crystalline phase.
Other conducting polymers interesting for their optical properties are oxidized polythiophenes and polypyrroles.
- "Electrical and electrochemical properties of conducting polymers" T.H. Le, Y. Kim, and H. Yoon, Polymer 9 (2017) 150 [DOI: 10.3390/polym9040150].