Self assembly on solid surfaces
There are numerous examples of self-assembly of molecules adsorbed on a solid surface (silicon, metal surface, graphene ...). The regular pattern formed by the molecules is the result of their mutual, non-covalent interactions. The surface and the medium underneath may also play a role in the supramolecular organization.
|
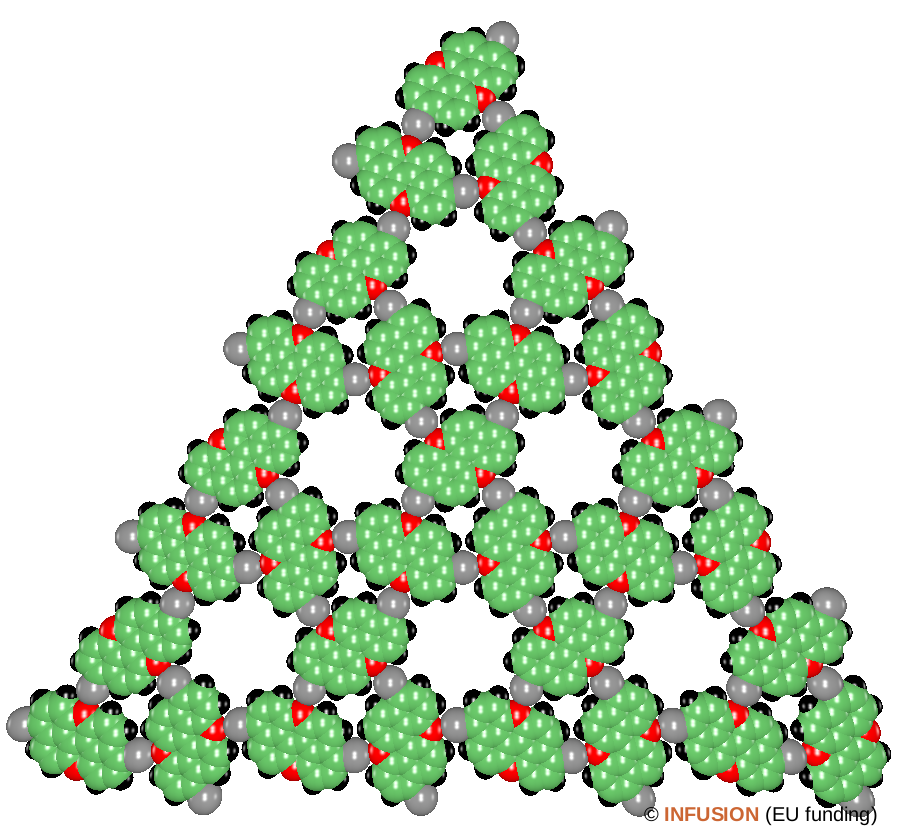
The figure on the right is a computer rendering (space-filling model) of the ordering of brominated polycyclic aromatic molecules observed by STM on a Au(111) surface. The molecules under investigation are bromine-doped peri-xanthenoxanthene (PXX). The latter compound, with formula C20O2H10, contains two rows of three hexagonal rings each, where the C atoms at the center of the two external zigzag chains are substituted by O (red spheres). Br2-PXX follows from it by Br substation for two opposite H atoms (gray spheres). The molecules assemble onto a chiral Kagome structure formed by a triangular lattice with 2.2-nm lattice parameter. Each unit cell contains three Br2-PXX molecules aligned along the edges of a regular triangle [1]. This arrangement generates big holes at the center of hexagonal rings of molecules, where the name Kagome structure comes from. The resolution of STM can be improved by working at low temperature with a CO-functionalized tip. High-resolution imaging obtained in that way indicates that the observed structure is driven by halogen bonding resulting primarily from electrostatic forces. It means that the Br atoms of each molecule are facing an O atom of a neighboring molecule, the Br and O atoms acting as donor and acceptor, respectively. The measured O···Br distance is 0.3 nm, slightly less than the sum of the van der Waals radii of the two atoms, which points to halogen bonding. This finding is confirmed by state-of-the-art electronic structure calculations. |
 |
- "Combining high-resolution scanning tunnelling microscopy and first-principles simulations to identify halogen bonding" J. Lawrence, G.C. Sosso, L. Đorđević, H. Pinfold, D. Bonifazi, and G. Costantini, Nature Comm. 11 (2020) 2103 [DOI: 10.1038/s41467-020-15898-2].