Pigments and phosphors
A pigment is a substance that absorbs light unevenly across the visible spectrum. Radiations for which the absorbance is large are less transmitted and reflected than those which are weakly absorbed. As a result, the substance acquires a color under white light illumination. When the pigment possesses a well marked absorption band, it is perceived with a color complementary to the one corresponding to the absorption band. Inorganic pigments have a simple chemical structure. They contain minerals, often in the form of metal compounds. Organic pigments have much more complex structures thanks to which almost infinitely many colors can be realized. Alizarin is one of the earliest used natural organic red pigment, extracted from plants and nowadays produced by chemical synthesis. Other examples of organic pigments or dyes can be found in the section devoted to chromophores.
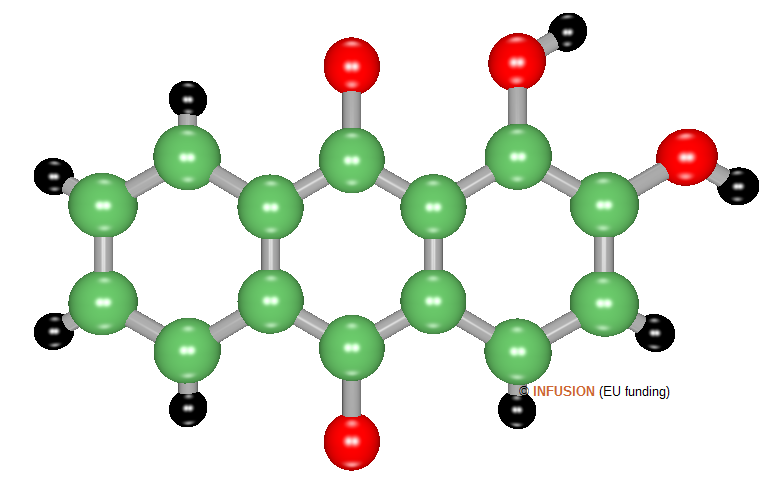 |
Ball-and-stick model of alizarin, with chemical formula C14H8O4. It is a planar aromatic molecule containing two hydroxyl group. A solution of alizarin in methanol has an absorption band centered around 430 nm due to HOMO-LUMO transition [1]. |
The biological world uses special organic pigments. Melanin is the principal pigment of human skin and hair. Its absorbance decreases monotonously and roughly exponentially with wavelength between 400 and 800 nm. The color of hairs depends mainly on its melanin concentration, black hairs containing more melanin than blond hairs. Chlorophyll has two strong absorption bands located respectively in the blue and red parts of the spectrum. It weakly absorbs light between 500 and 550 nm, and therefore appears green
Phosphor is a generic name for substances that can transform light color by photoluminescence. In this process, a fraction of absorbed visible or UV radiations are re-emitted at longer wavelengths. The distinction between phosphorescence and luminescence mechanisms is chiefly the time over which light re-emission survives after absorption. Phosphors are widely used in color displays, in fluorescence lamps and in LED lighting, e.g. to transform the dominant blue light emitted by InGaN into white light. Many inorganic compounds can be used as phosphors. In general, they need to be doped with an activator (alkali atoms, Cu or Ag) to reach high efficiency. Many organic molecules are luminescent, perhaps the most well-known example being fluorescein.
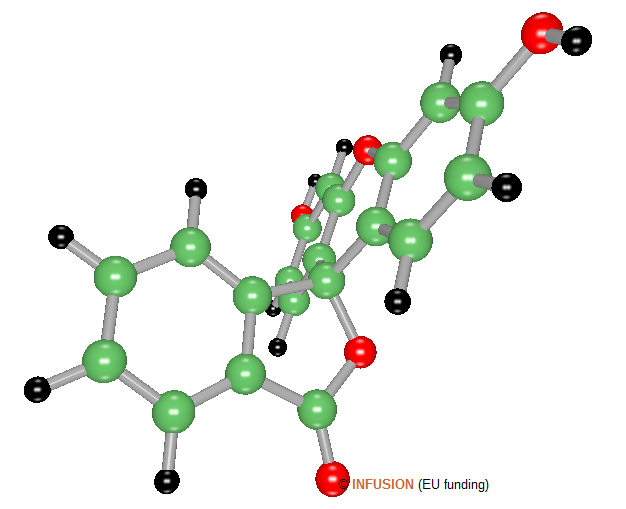 |
Ball-and-stick model of a 3D conformer of fluorescein, with chemical formula C20H12O5. In solution, this molecule absorbs light mainly in the blue and emit light mainly in the green (at 540 nm in ethanol, 521 nm in water). Derivatives of fluorescein are commonly used to mark cells for fluorescence microscopy investigations. |
- "Color prediction from first principle quantum chemistry computations: a case of alizarin dissolved in methanol" P. Cysewski, T. Jelinski, M. Przyby, and A. Shyichuk, New J.Chem. 36 (2012) 1836 [DOI: 10.1039/C2NJ40327G].